 http://www.wwf.it/summit/images/19061_germany%20Acid%20rain.jpg
http://www.wwf.it/summit/images/19061_germany%20Acid%20rain.jpg
Acid rain, or more generally acid deposition, is defined as any precipitation with a pH below 5.6 (or more appropriately below 5.0). A more accurate term is acid deposition. Wet deposition occurs when pollutants are carried in rain, snow, mist and low cloud; pollutants may be wet-deposited after being carried long distances. Dry deposition is the direct fallout of acid pollutants and is present mostly in areas close to the source of emission.
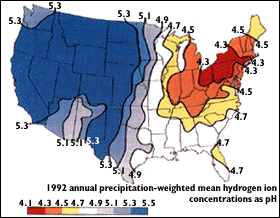 http://pubs.usgs.gov/gip/acidrain/gifs/fig04.gif
http://pubs.usgs.gov/gip/acidrain/gifs/fig04.gif
The above map of the United States shows that the region with the greatest acidity level is the Northeast. The high acidity is caused by the large number of cities, the dense population, and the concentration of power and industrial plants. In addition, the wind direction brings storms and pollution from the Midwest to the Northeast. The map below shows the prevalence of acid rain in the world. It is clear that the more industrious and heavily populated areas, such as parts of the U.S., Europe and eastern China, are more prone to acid rain.
 http://go.hrw.com/venus_images/0647MC30.gif]
http://go.hrw.com/venus_images/0647MC30.gif]
The two main components of acid rain are sulfur dioxide (SO2) and nitrogen oxides (NOx).
Sulfur dioxide is a colorless gas released by combusting fossil fuels that contain sulfur. In the United States, 67% of SO2 emission comes from electric utilities, 28% from fuel combustion and industrial sources, 5% from transportation, and 1% from natural disasters.
 http://news.bbc.co.uk/media/images/38261000/jpg/_38261905_carexhaust300.jpg
http://news.bbc.co.uk/media/images/38261000/jpg/_38261905_carexhaust300.jpg
Nitrogen oxide, a term used to describe any compound of nitrogen with any amount of oxygen atoms (for example, nitrogen monoxide NO and nitrogen dioxide NO2), is the other chief component responsible for acid rain. In the United States, 54% of these gases are released by automobiles and utility plants, 22% by electric utilities, 22% by fuel combustion and industrial sources, and 2% from natural processes. Lastly, 5% of nitrogen oxide emissions are produced through natural processes such as decomposition, forest fires, volcanoes, and lightening. Nitrogen oxide is capable of causing respiratory illness and contributes to ozone damage.
 http://www.dec.state.ny.us/website/dar/ood/acidrain.gif
http://www.dec.state.ny.us/website/dar/ood/acidrain.gif
The most common process that sulfur dioxide undergoes is when it reacts with moisture in the atmosphere and immediately oxidizes to form sulfur trioxide.
SO2(g) + O2(g) --> SO3(g)
Then, the sulfur trioxide joins water in forming sulfuric acid.
SO3(g) + H2O(l) --> H2SO4(aq)
Similar to sulfur dioxide, nitrogen oxides in the atmosphere are oxidized to form nitric or nitrous acid. The following is the formation of nitric acid from nitrogen dioxide and water in the atmosphere.
3NO2(g) + H2O --> 2HNO3(aq) + NO(g)
However, not all of these gases are converted to acids. A significant amount float up into the atmosphere, is carried to another area hundreds of miles away, and is deposited on the earth unconverted. When they are converted and fall back to the earth through condensation, these acids not only cause irreparable damage to living things, but also to architectural structures. Learn more about various prevention methods and what you can do to fight acid rain.
♣Top♣
♥Home♥
Bibliography
Phamornsuwana, Sarn. "Causes, Effects, and Solutions of Acid Rain". Online. http://www.geocities.com/CapeCanaveral/Hall/9111/DOC.HTML May 25, 2006.
"The Green Lane: Acid Rain and the Facts". Online. http://www.ec.gc.ca/acidrain/acidfact.html. May 25, 2006.
Comments (1)
Anonymous said
at 5:46 am on May 20, 2006
Excellent introduction and summary. Please check the chemical reactions: Sulfite is SO3, minus 1. SO3 without a charge is sulfur trioxide. I'm not sure which is correct, but the equation has sulfur trioxide and the words say sulfite. In the NOx reaction, is the reactant hydroxide ION (minus 1 charge) or hydroxide RADICAL? A radical is a neutral, reactive species with an unpaired electron, that would be written as a dot. Both might be referred to as hydroxide. Please check this and correct your equations and/or text.
You don't have permission to comment on this page.